Safe battery solutions for medical technology
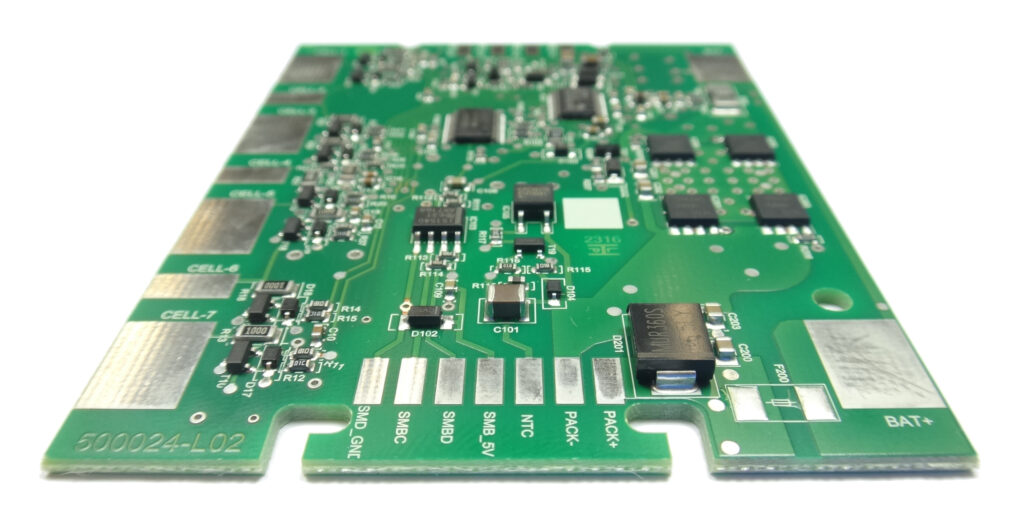
Engineering and production of customized lithium-ion battery packs require the creation of a reliable overall system in accordance with the battery management system (BMS) used. As long-term supplier for medical technology FRIWO is well aware of the importance of maximum safety and reliability. Failures of battery packs may entail dire consequences if, for instance, respiratory devices or defibrillators are powered by them. Accordingly increased demands are placed on the electronics of a battery pack: power consumption, pulse load, measurement precision, temperature monitoring and long runtimes are essential parameters which must be considered when designing a BMS for medical products.
Basic structure of a battery management system
The architecture of a battery management system can be composed from a variety of different functional modules, depending on the specific requirements and respective runtime expectations of the lithium-ion battery. The design of the BMS is significantly determined by the ICs which are to optimize the runtime of the battery. In addition to the basic protection against overvoltage and undervoltage, overcurrent, short circuit and overtemperature, various other function blocks can also be integrated. For example, parameters for charge and discharge are stored in the microcontroller of the BMS.
Integration of two-tier safety concepts
In addition to the conventional protective features, a two-tier safety concept should be implemented for medical battery management systems to ensure the highest possible patient and handling safety. The first safety tier is usually implemented by FRIWO using a MOSFET circuit. Charging and discharging MOSFETs are arranged back-to-back since, in case of emergency, a current interruption in both directions must be possible. Various MOSFET circuit topologies are available for implementing this first safety tier, allowing an optimization of the respective BMS in terms of power consumption and other requirements. Another safety tier, the so-called 2nd protection, can be implemented, for example, by integrating a fuse in addition to the MOSFET.
Ideal lifespan with cell balancing
Not only the battery pack’s safety but also its lifespan plays an important role in medical technology. The so-called “cell balancing” is a life-prolonging feature which offers advantages for the series connection of cells. In this process, the voltage level of the individual cells is measured and, in case of deviations, adjusted by individual cell loading. As a result, all cells have an identical level even after many cycles which positively affects the lifespan of the entire battery pack.
Communication options of a battery pack
Except for safety and lifespan features, a variety of communication options can also be implemented with a corresponding BMS. Matching interfaces allow an information exchange with the charger for an optimal charging process. Furthermore, the medical device or the user can be provided with manifold information on the battery pack and charging process like, for instance, remaining runtime or maximum available battery capacity. Even individual cell voltages, remaining charging time, temperature or active current can be communicated. FRIWO, for example, uses SM bus interfaces for such data transfers. The SM bus is based on the popular I2C bus protocol which is used for communication between different modules. The bit rate on this two-wire bus can reach up to 100 Kbit/s allowing fast data exchange.
Accurate display of remaining battery runtimes by various methods
In medical technology, the battery’s remaining runtime is of utmost importance since a single field failure could have drastic consequences. All the more important is an accurate and reliable output of this status. This can be done in a number of ways but the “impedance track gas gauge” procedure has prevailed. In this method, the measurement of the battery impedance, which changes with increasing charge and discharge, is combined with a regular measurement of open circuit voltage. FRIWO also increasingly uses the CEDV (Compensated End of Discharge Voltage) algorithm. The determination of voltage values for different loads and the consideration of cell chemistry and number of cycles characterize this scheme.
Protection against piracy
Plagiarism protection plays an essential role for maximum operational safety of medical devices. FRIWO’s intelligent lithium-ion battery packs can therefore be equipped with an SHA-1 authentication key which ensures the utilization of original batteries by the user. Consequently, the first communication between battery pack and application consists of identifying the battery’s authentication key. If the medical device confirms the key, the battery can be used. Otherwise it will be rejected. The authentication key can, for example, be identical for all standard batteries of a manufacturer or can be customized.
Exact alignment of the BMS with each application
When engineering a BMS, the abundance of possible feature combinations is almost unlimited. For an optimally functioning system, the features should always be precisely combined in accordance with each application’s requirements, as each application has different requirements in terms of runtime, safety and communication options of a battery pack. For customized BMS solutions FRIWO offers a vast engineering know-how at its headquarters in Ostbevern/Westphalia. Here, under the quality hallmark “Made in Germany”, complex battery management systems with communication and parameter queries can be implemented.
Are you interested in our medical products and application solutions?
Find out more HERE.